Aldol Condensation Lab Report Abstract
Synthesis of dibenzalacetone using the.

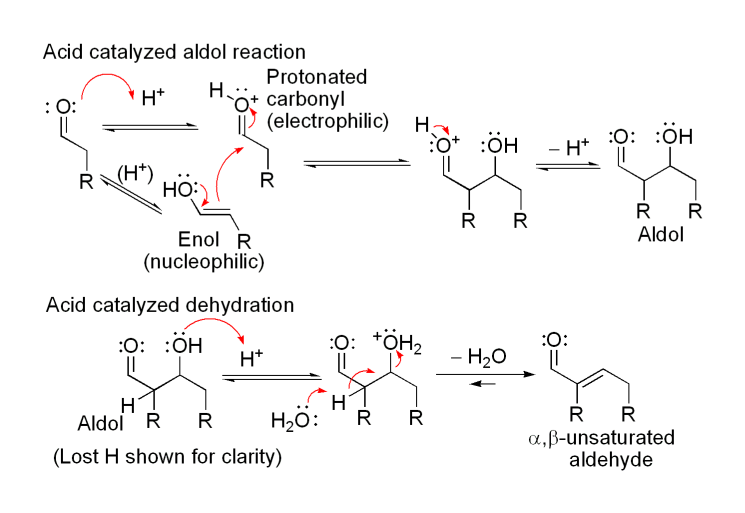
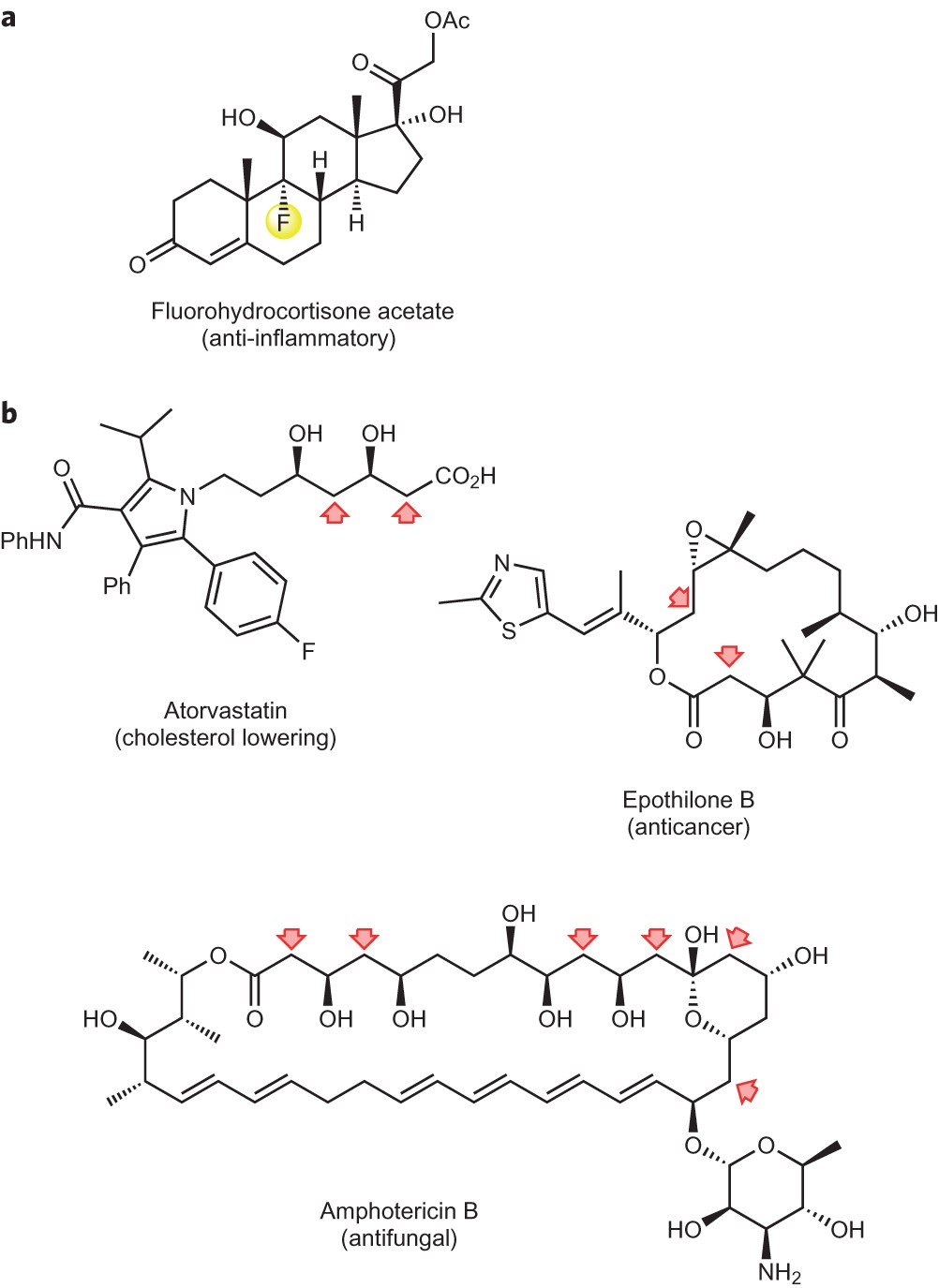
Aldol condensation lab report abstract. The product that we obtained in our percent yield is 69 and we obtained 559 g of crystal back. An aldol condensation is a condensation reaction in organic chemistry in which an enol or an enolate ion reacts with a carbonyl compound to form a b hydroxyaldehyde or b hydroxyketone followed by dehydration to give a conjugated enone. The precipitate formed during this step was weighed and the melting point was recorded. The experimental procedure followed the format referenced in the lab manual chemical education resources.
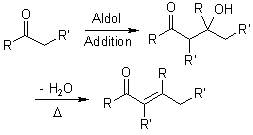
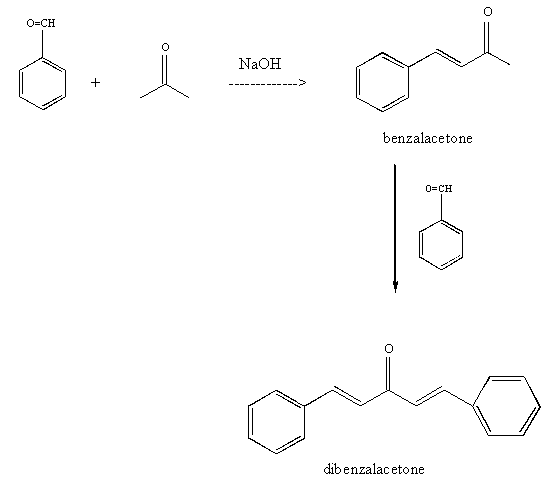
The melting point that was observed was 1100c which is equal to the trans trans isomer. One of the key reactions used the aldol condensation features the reaction of two carbonyl compounds to form a new b hydroxy carbonyl compound1. In this particular reaction with benzaldehyde and acetone are combined to effectively lose water. Aldol condensation synthesis of dibenzalacetone pre lab report guidelines the synthesis of dibenzalacetone period 1 is done individually in preperation for lab your prelab report should include the following 1 introduction in this section outline the goal s of the experiment as well as the experimental techniques that you will be using to purify and isolate the desired product s 2 prepare a half page flow chart of the experimental design indicate on your flow chart a time plan for the.
Reaction between acetone and benzaldehyde. An aldol condensation reaction is one in which an enol or an enolate ion reacts with a carbonyl compound to form b hydroxyaldehyde or a b hydroxyketone hence a dehydration gives a conjugate enone. Aldol condensation is different from aldol condensation reaction. Acetone and benzaldehyde were.
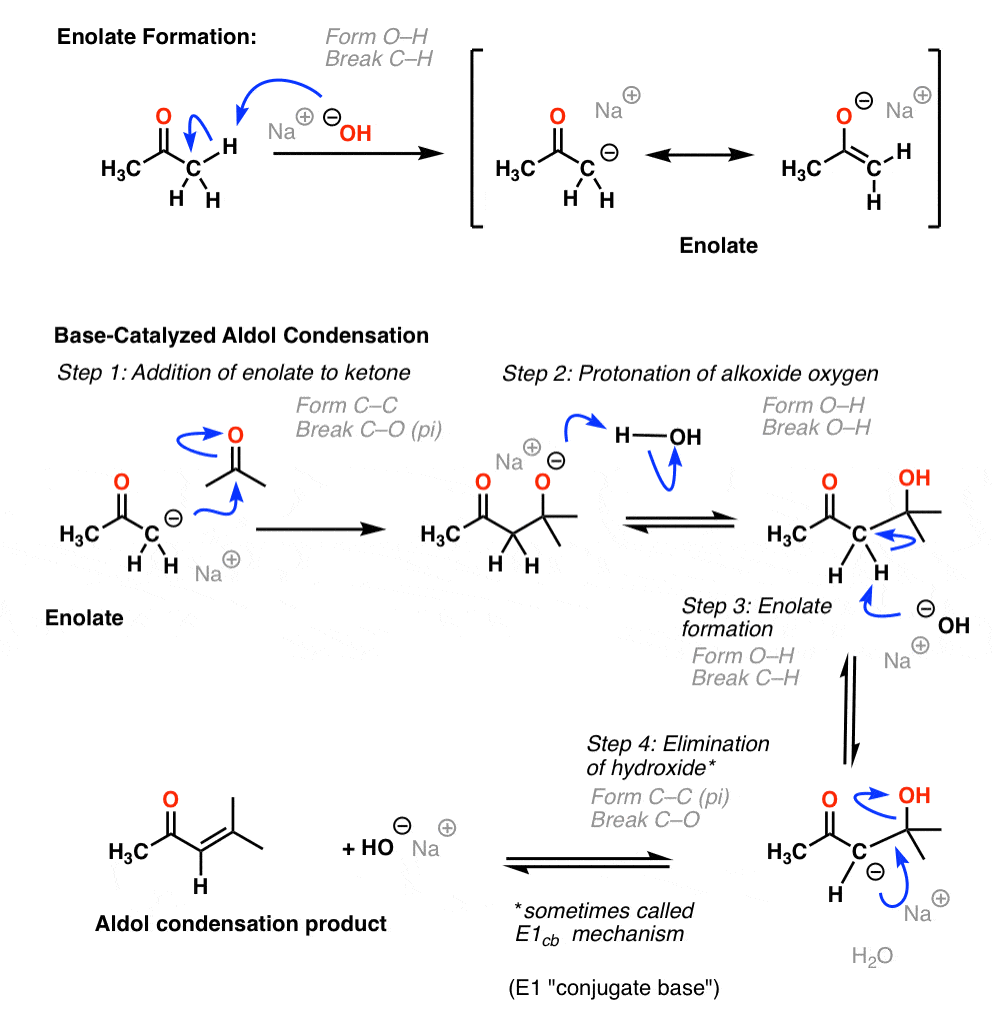
Mixed with sodium hydroxide and ethanol and mixed vigorously for 30 minutes. The only experiment performed with the assistance of this lab manual was on page 101 103 semi microscale aldol condensation. Aldol condensation is a c c bond forming reaction between the alpha carbon of the aldehyde or ketone and carbonyl carbon of another ketone or aldehyde. The reaction began by creating a carbanion intermediate.
Discussion b the aldol condensation reaction is a base catalyzed reaction in which two larger molecules are added and a smaller molecule is lost. For aldol addition reaction the alpha carbon of one aldehyde or ketone adds to the carbonyl group of the other ketone or aldehyde. In this reaction we obtained the correct melting point of 106 0 c which is 2 degrees off what is said in the msds. In both parts of this practical experiment benzaldehyde reacts with a ketone in the presence of a base to.
In this experiment dibenzalacetone was prepared via an aldol condensation. This reaction can be performed under acid or base catalyzed conditions and usually results in the formation of an bb unsaturated carbonyl compound.