Chemical Kinetics Lab Report Abstract
Standards were used to identify unknown amino acids in a mixture.
Chemical kinetics lab report abstract. Run several experiments at different initial concentrations to see how the. Chemical kinetics experiment 3 abstract results discussion and appendix. Reactions in which large molecules are formed or break apart are usually slow. A b c d the rate of reaction is measured by observing the rate of disappearance of the reactants a or b or the rate of appearance of the products c or d.
Lab report rate of chemical reaction. Chemical kinetics deals with the speed or rate of a reaction and the mechanism by which the reaction occurs. From these values the activation energy e a can be determined. Abstract in this experiment chromatography was used to analyse amino acids in solution.
Ascending layer chromatography with an isopropanol based solvent was used to separate the amino acids which were then detected with ninhydrin. Rate constants and activation energy purpose. Different rates depending on the nature of its reactants the concentration of the substances the. Chemical kinetics is the branch of chemistry that is concerned with the study of the rate of chemical reactions.
Temperature of the substances and the presence of catalysts. Time indicates first order kinetics and a straight. An example abstract from a chemistry report. Rinaldi 1 chemical kinetics.
The purpose of this lab is to examine the rate of a first order chemical reaction at two different temperatures to determine the rate constant of the reaction at each temperature. The iodination of acetone members section 2. Whereas reactions breaking strong covalent bonds are also slow. Include a copy of the printout of your graph with your lab report.
Constants of the rate equations. Rates of chemical reactions are measured through chemical kinetics. Iii1 the iodine clock reaction. A linear plot of ln dye vs.
By adjusting the concentration of reactants in each mixture and recording times of chemical reaction we would be able to find the orders and the rate. The higher the concentration and the temperature of the solution are the faster the reaction will be. Chemical kinetics consider the hypothetical reaction. This study aims to cast light on the.


















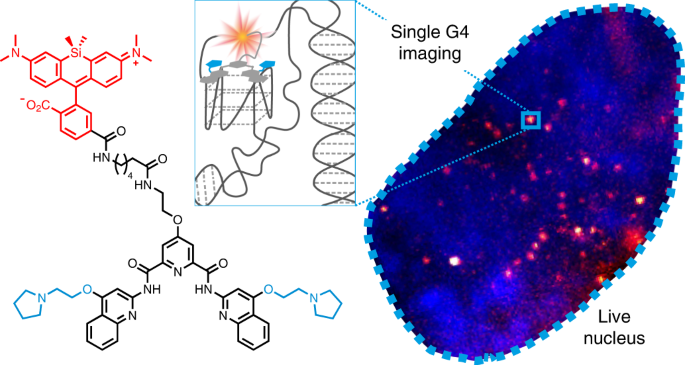
































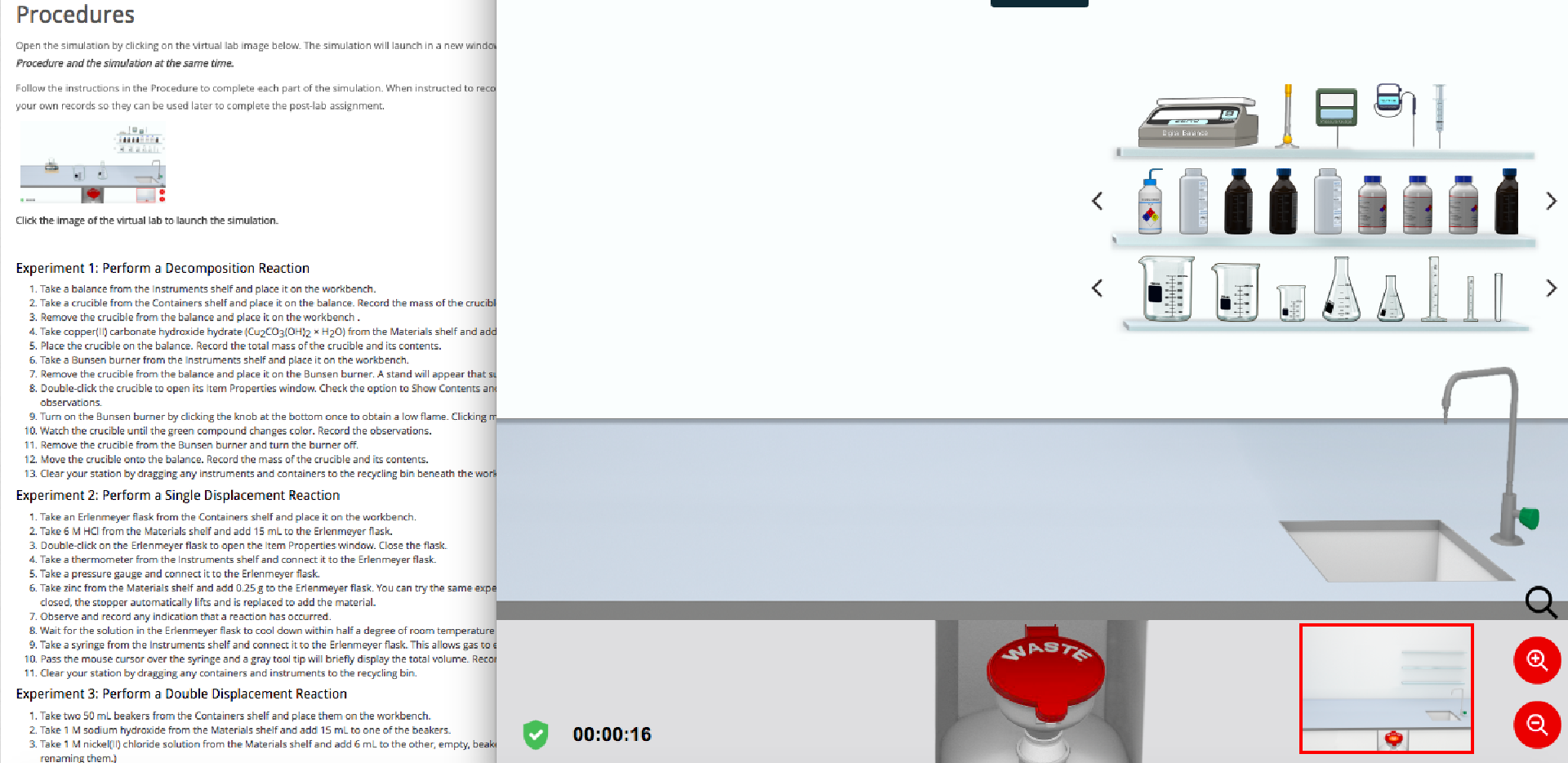










.PNG)





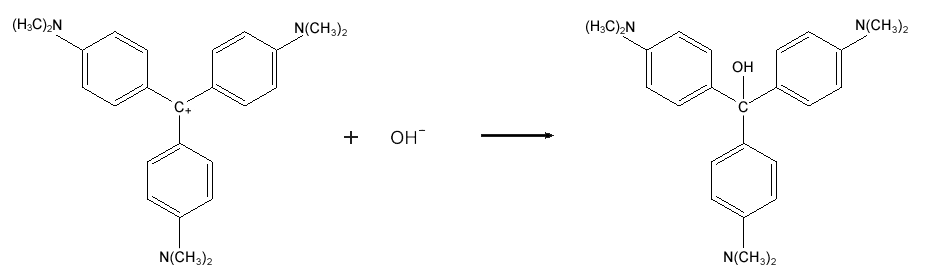

.PNG)