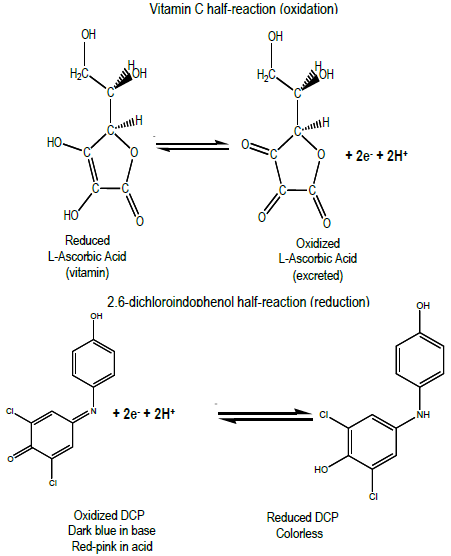
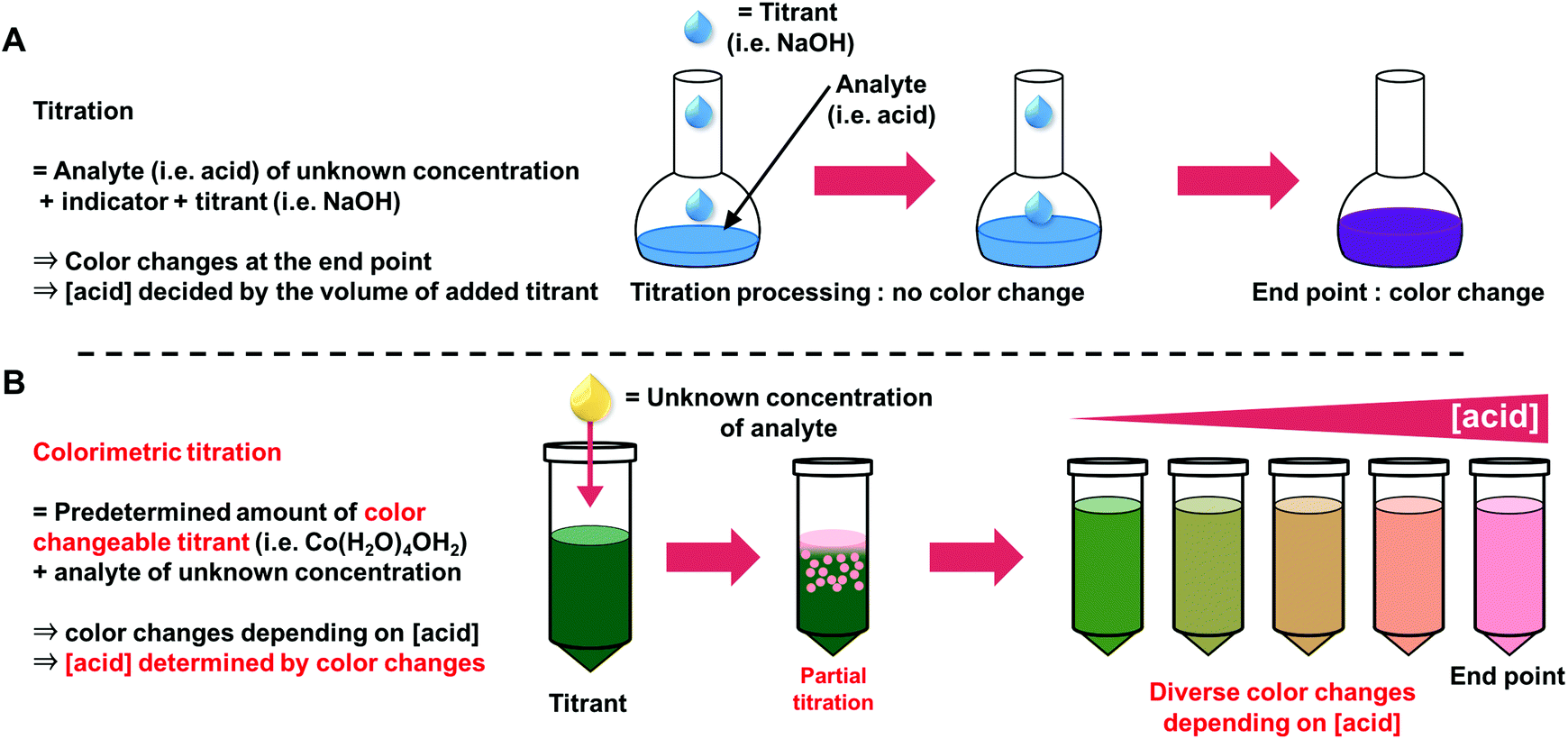
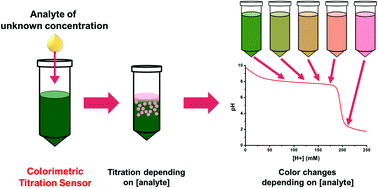
Acid Base Titration Lab Report Abstract
The purpose of this experiment is to observe the titration of hydrochloric acid astrong acid with sodium hydroxide a strong base and acetic acid a weak acid with sodiumhydroxide a strong base.

Acid base titration lab report abstract. Concentration is titrated with a solution of sodium hydroxide of 01 molarity. In order to do this we will be titrating a known molarity of naoh into khp with an indicator and doing twice. Bases would be titrated with an acid and acids would be titrated with a base. By observing the titration of a strong acid and strong base and a strongbase and weak acid one can see how the shapes in the titration curves differ.
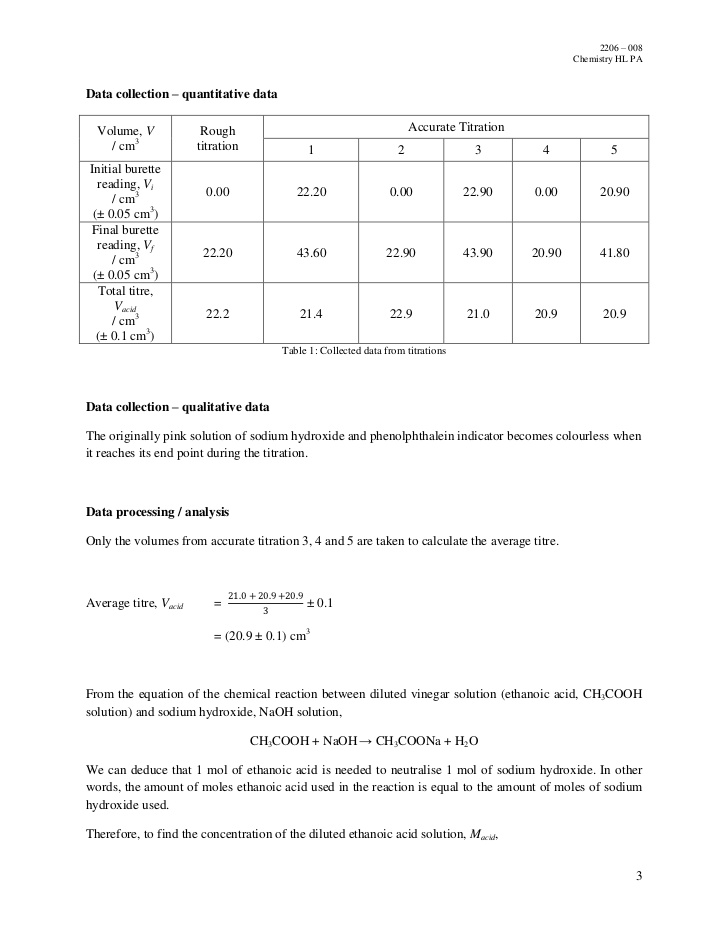
A reader employs the abstract to rapidly understand the objective. The purpose of this titration lab is to determine the molarity of the given acetic acid. At the point where there is a vertical asymptote in the graph produced by the titration the solution is neutral. Experiment 1011part 1 acid base titration.
Acid and base titrations lab report chm 114 jx abstract this goal was to give us experience finding the standardization of through the use of a primary standard. Experiment 6 acid base titration lab report include these parts in the pre lab for meeting 2 the purpose of this experiment is to determine the concentration of a solution of sodium hydroxide by titration against a standard so for ph 7 the ceh ion concentration is 10 7 m the ph values of everyday chemicals. The solution completely neutralizes. In this lab a solution of acetic acid of unknown.
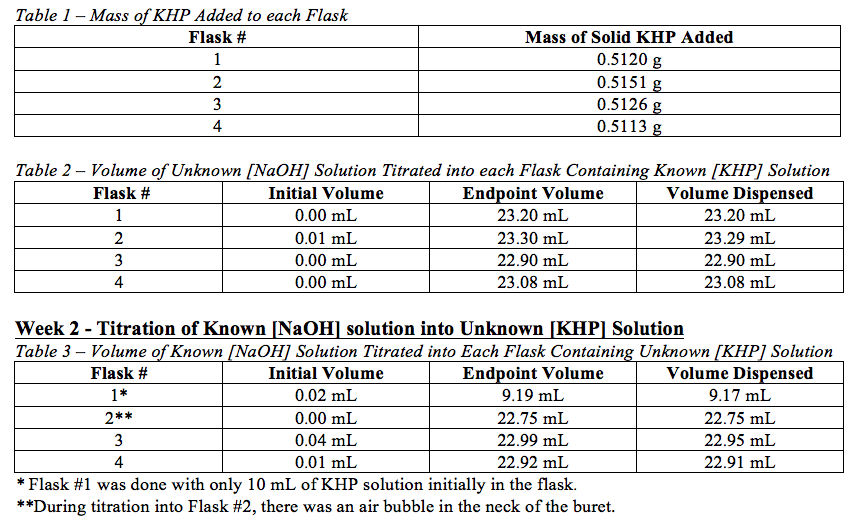
Abstract the concentration of the base sodium hydroxide naoh was determined through the use of a laboratory procedure called titration by standardizing it against a weak acid using a phenolphthalein end point. In this experiment we will be using naoh and hcl as well as khp. Acid base titrations can offer valuable information concerning the nature. The result was an average naoh molarity of 91 05m.
Introduction in this lab the objective was to standardize or to determine the concentration of a sodium hydroxide solution naoh using a solution of the weak acid potassium hydrogen phthalate khp through the process of. Abstract by using acid base titration we determined the suitability of phenolphthalein and methyl red as acid base indicators. Demonstrates how the molarity of an acid can be determined by titrating an acid with a base until.