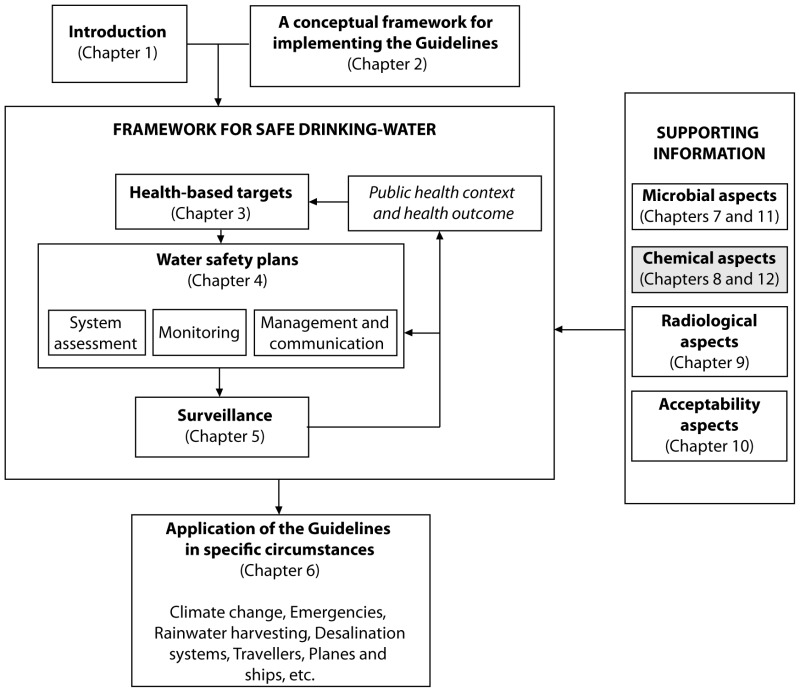
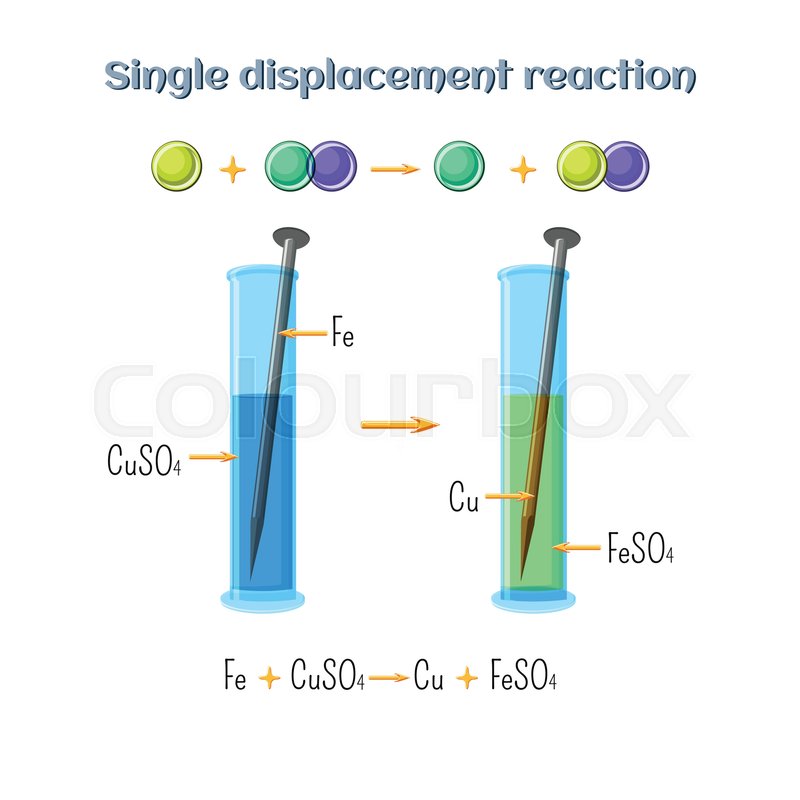
Abstract For Reaction Of Iron With Copper Sulphate Solution
As we know the colour copper sulphate solution is blue in colour.
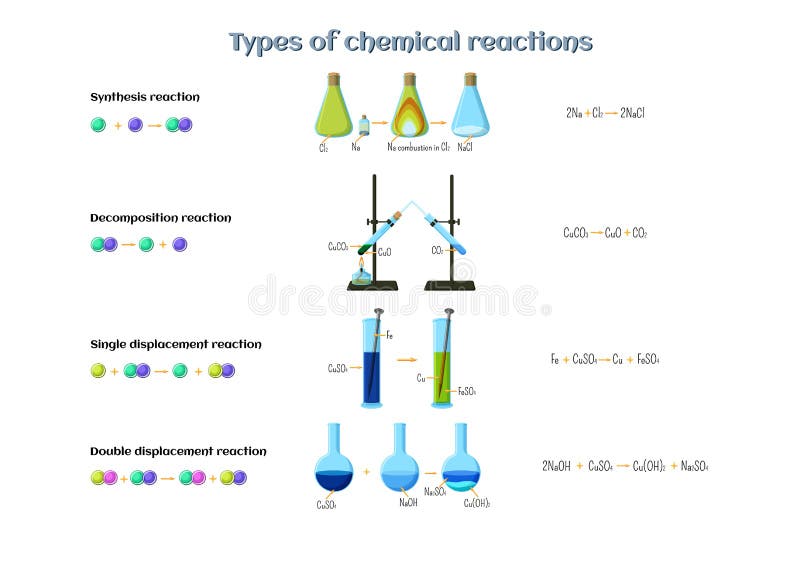
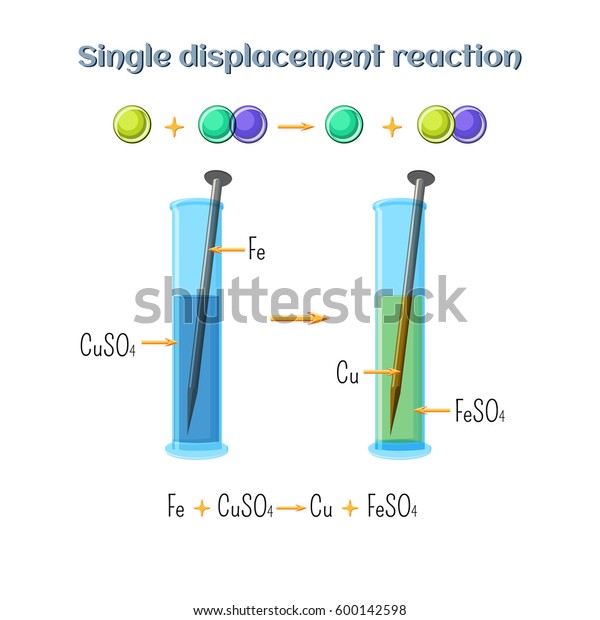
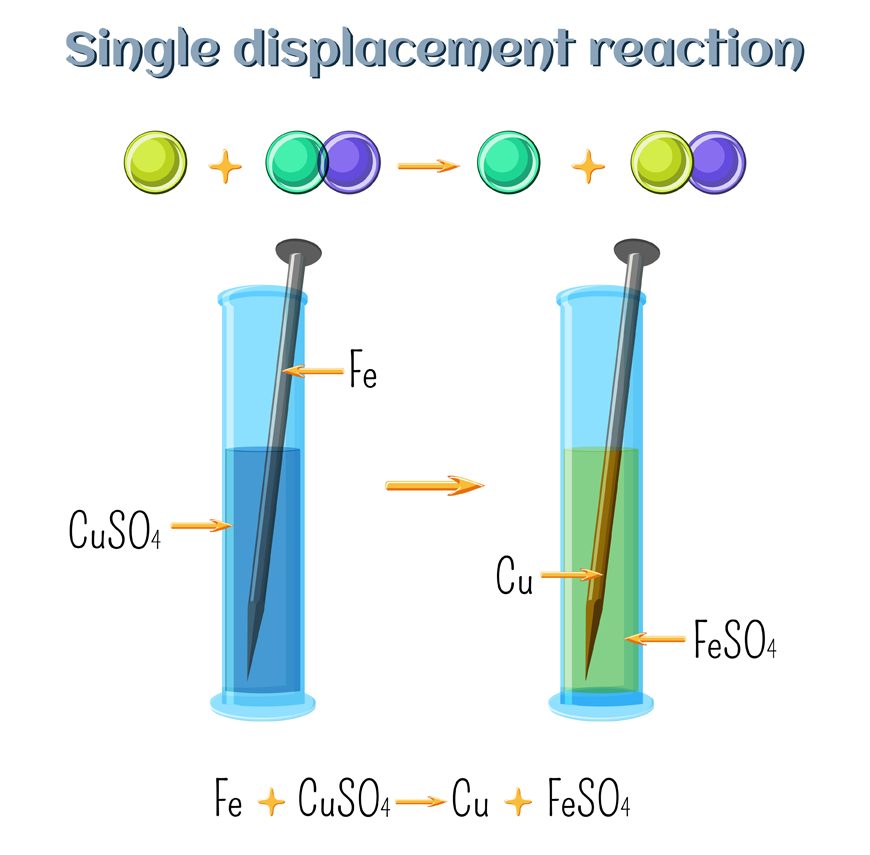
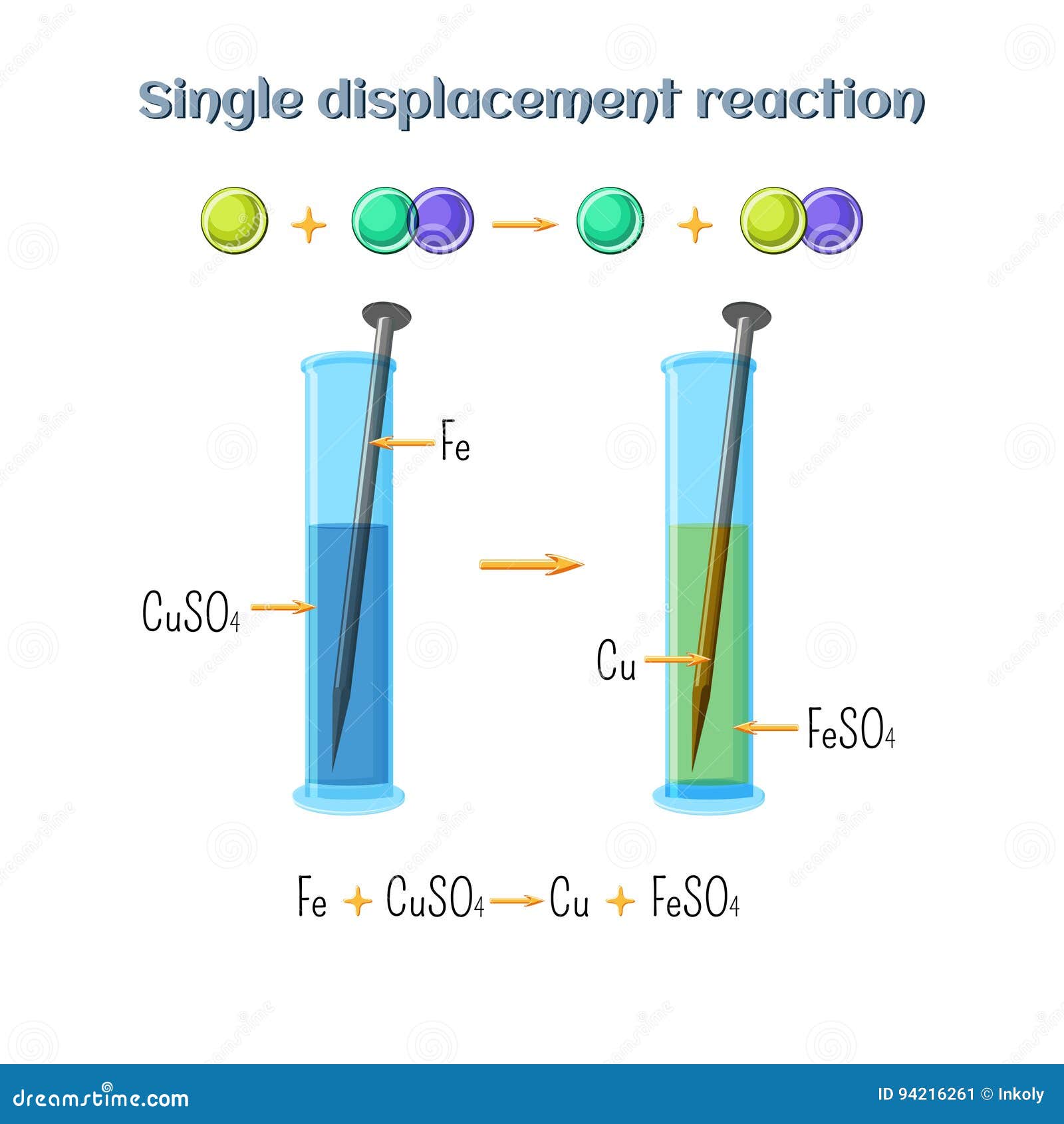
Abstract for reaction of iron with copper sulphate solution. Iron in nail displaces copper from the copper sulphate solution. When solid copper is reacted with a silver nitrate solution two reactions are possible as shown in the following equations. The iron dissolves turning into solution and metallic copper of a reddish color is released. This is an example of a displacement reaction.
Ags cuno3 aq ag1aq cus. Agno3 aq cus. So this reaction is a chemical change. This is a single displacement reaction in which copper has been displaced by iron from copper sulphate solution and a new compound ferrous sulphate is formed.
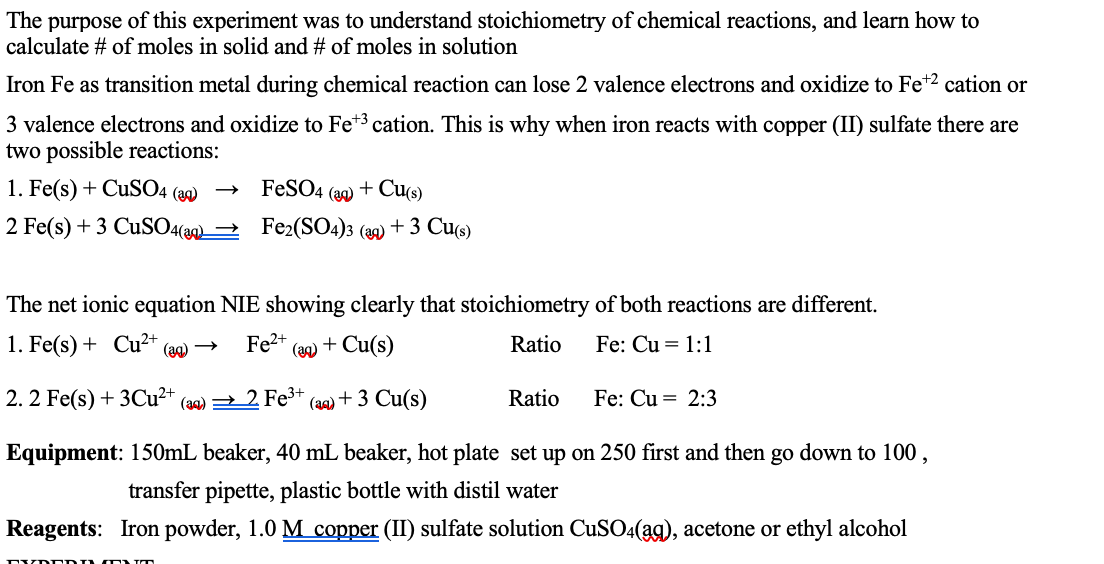
Cuso 4 aq fe s feso 4 aq cu s note. The iron atoms have now changed into iron 3 ions. Ags cu1aq b. The copper metal is now in the element form the reddish brown copper element in the test tube is forced out of the solution and coats the iron filings.
This is the reaction that is used in extraction of copper. Fe s cuso 4 aq feso 4 aq cu s 4. The chemical equation for this reaction is as follows. Elemental copper deposits on the nail and impart a brown colour to the nail.
The reaction of iron with copper ii sulfate copper can form two possible cations cuprous cu1 and cupric cu2. Answer iron being more reactive than copper will displace copper from its salt and form a subsequent salt of ferrous sulphate. The iron atoms give electrons to make the copper 2 ions. Iron is more reactive than copper and this is the reason why iron displaces copper in the copper sulphate solution.
The solutions colour also changes to light green colour. When we put iron nail in it copper forms brown coloured coating on iron nail. Hence it is a displacement reaction as iron is more reactive than copper and copper is displaced to form ferrous sulphate solution. During the reaction the colour of copper sulphate blue will change to greenish blue ferrous sulphate.
2agno3 aq cus. This then produces a solution of iron sulfate which is the green solution. As a result solution fades in colour. The faded blue apperance later change to green colour because ferrous sulphate is green.
The iron in the container displaces the copper in the solution and thus it.