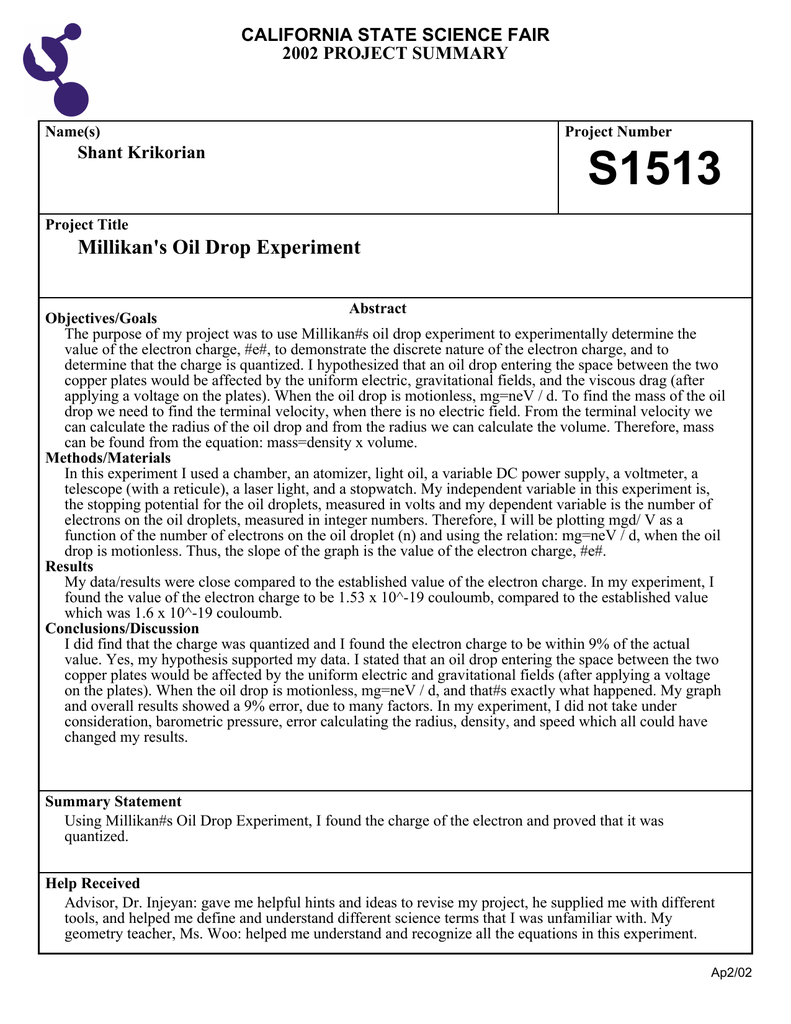
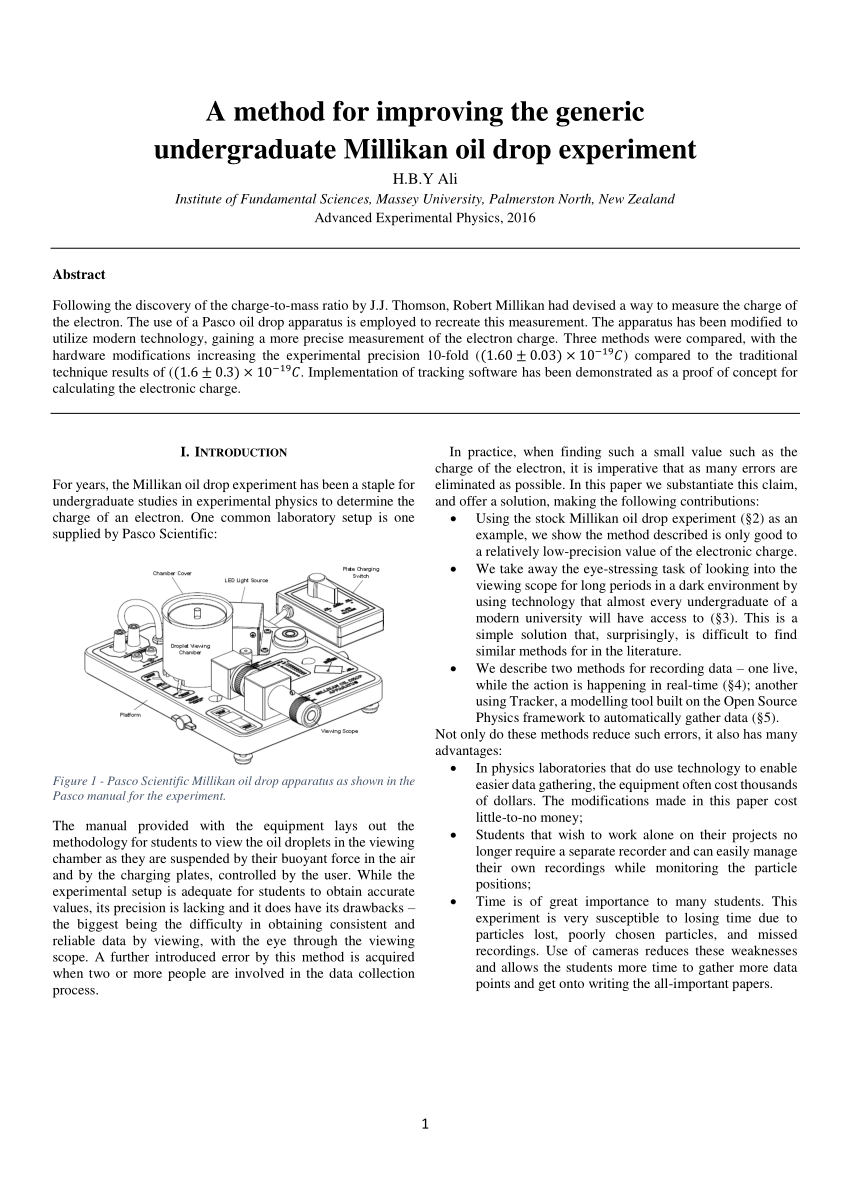
Abstract For Millikan Oil Drop Experiment
In 1909 robert millikan and harvey fletcher conducted the oil drop experiment to determine the charge of an electron.

Abstract for millikan oil drop experiment. It was first performed in a 1909 by robert a. Accurate timing of their motion becomes possible since they are now bright and sharply focused. The purpose of an abstract is to provide enough information to the reader that he or she can decide whether to read the rest of the article. Abstractthe experiment was devised by american physicist robert a.
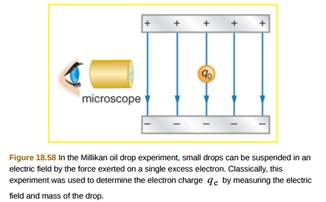
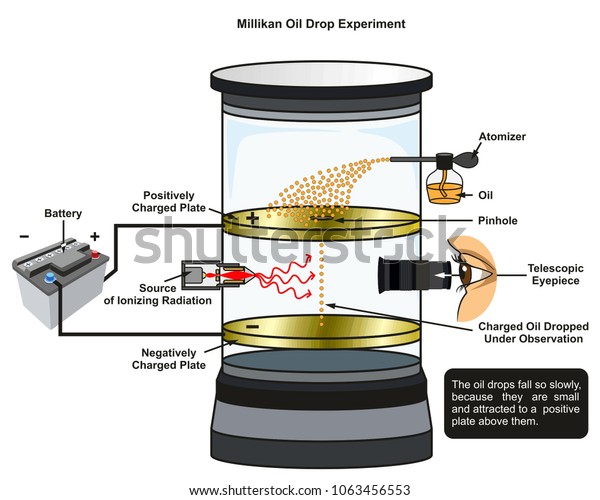
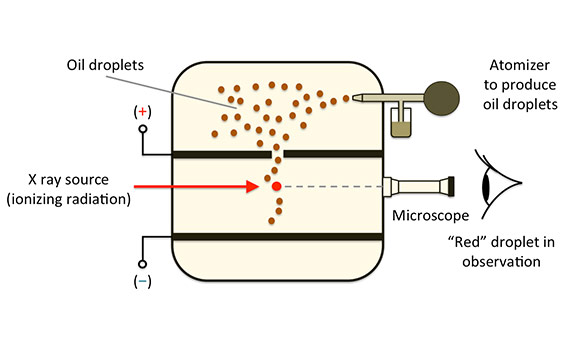
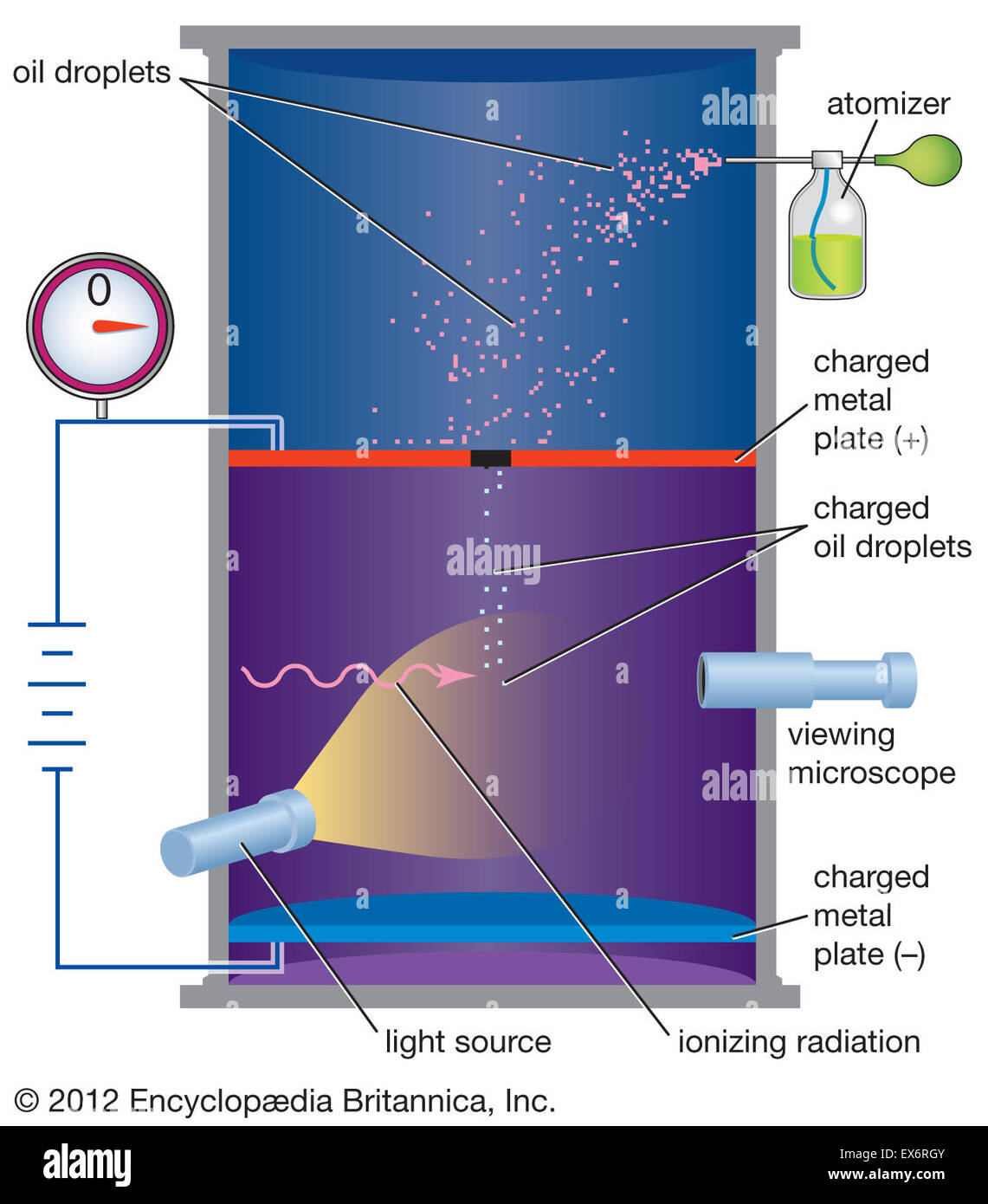
They suspended tiny charged droplets of oil between two metal electrodes by balancing downward gravitational force with upward drag and electric forces. The force on an electric charge in an electric field is equal to the product of the charge with the electric field. Enhancement of the optics in the oil drop apparatus greatly improves the visibility of the drops. For this work and for work on the photoelectric effect millikan was awarded the nobel prize in physics in 1923.
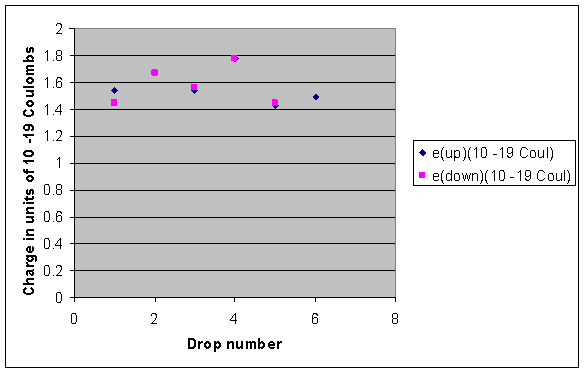
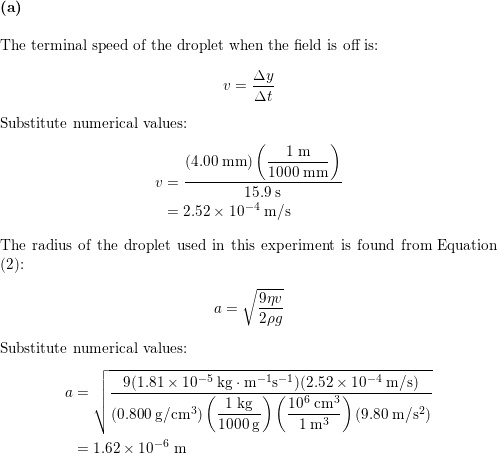
We used the classical method of millikan to measure the charge of the electron. Millikan wanted to determine whether electric charges occurred in discrete units which were integral multiples of some smallest charge e which turns out to be carried by an electron or proton and to measure that charge. He had previously published2other results with falling drops times between 12 to 19 seconds. Small charged oil droplets were observed to move with constant velocities under the influence of gravity and of applied electric fields of known values.
The oil drop experiment. Millikan oil drop experiment first direct and compelling measurement of the electric charge of a single electron. His best data was presented in his nobel prize acceptance speech1with an oil drop that fell 13 cm in a time of 120 seconds. Mea surements are made of the rise and fall times of oil drops illuminated by light from a helium neon laser.
It was performed originally in 1909 by the american physicist robert a. Millikan designed his famous experiment that was perfected over several years. The experiment a great improvement over previous attempts to measure the charge of an electron has been called one of the most beautiful in physics history but is also the source of allegations of scientific misconduct on millikans part. State the conceptual goal of the experiment the approach the result and the conclusion.
Millikan 1868 1953 began his experiments to measure the charge on the electron e in 1907. The experiments were performed in ryerson laboratory at the university of chicago where millikan was professor of physics. Revised 122708 millikan oil drop experiment advanced laboratory physics 407 university of wisconsin madison wisconsin 53706 abstract the charge of the electron is measured using the classic technique of millikan. The millikan oil drop experiment was the first compelling experiment that measured the charge of an electron.
It was then that ra.